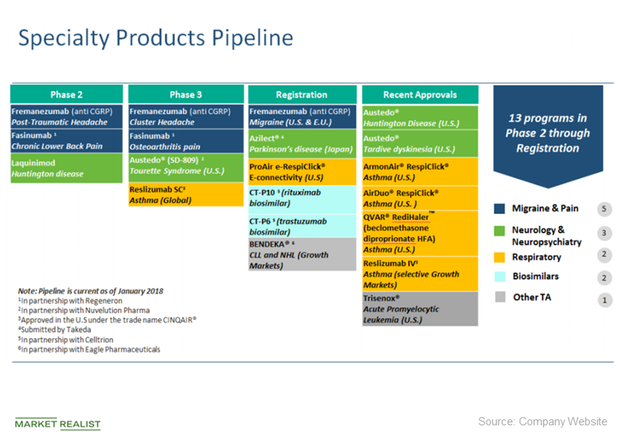
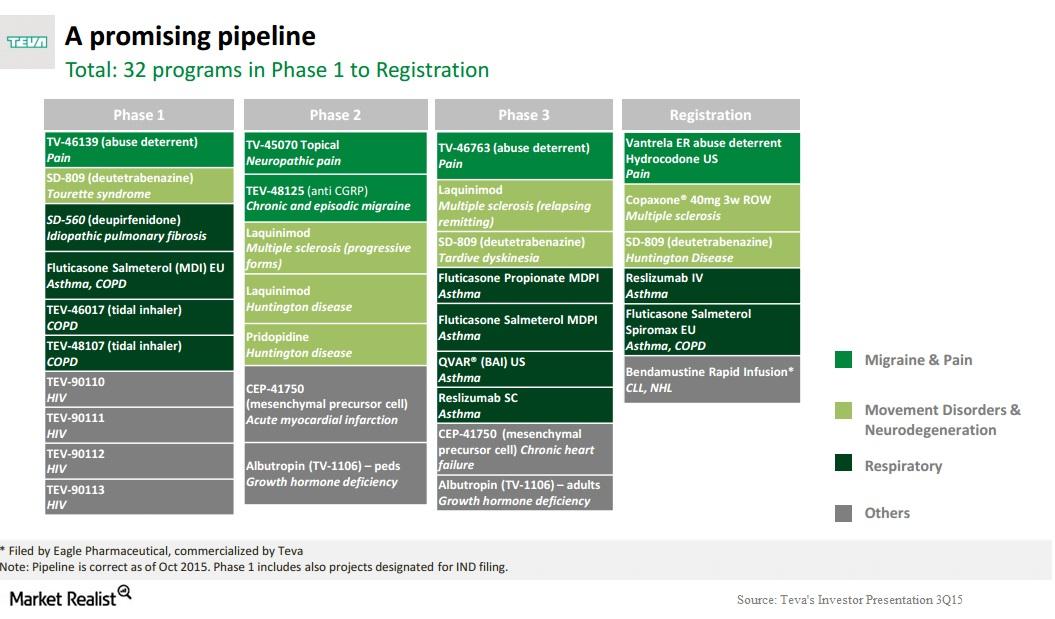
dough on Twitter: "@JohnCFierce $ACAD potential interested parties in $ACAD $BIIB $TEVA $AGN $PFE. IMHO I think it's $LLY https://t.co/QM6XZkoGwJ" / Twitter

ADDING MULTIMEDIA Teva Announces FDA Approval of First and Only Digital Inhaler with Built-In Sensors – ProAir® Digihaler™ (albuterol sulfate 117 mcg) Inhalation Powder | Business Wire
Teva's AJOVY® Receives EU Approval Offering Patients the First and Only Anti-CGRP Treatment with Both Quarterly and Monthly Dosing for the Prophylaxis of Migraine in Adults | Business Wire

Teva Announces FDA Approval of First and Only Digital Inhaler with Built-In Sensors – ProAir® Digihaler™ | SnackSafely.com

Teva Announces FDA Approval of AUSTEDO® XR (deutetrabenazine) Extended-Release Tablets, a New Once-Daily Formulation of AUSTEDO® (deutetrabenazine) Tablets | Business Wire

Teva's pursuit of J&J boosted by FDA acceptance of filing for approval of long-acting schizophrenia drug | Fierce Pharma

Teva Announces FDA Approval of AUSTEDO™ (deutetrabenazine) Tablets for the Treatment of Chorea Associated with Huntington's Disease — Hereditary Neurological Disease Centre